Abstract
Background and aims
Existing evidence regarding the effect of low-dose cyclophosphamide on immune cells in myeloma patients, in particular in combination with the IMiD® compound pomalidomide is limited. We present here for the first time changes in immune cell sub-group composition associated with the addition of cyclophosphamide to pomalidomide in the randomised MUKseven clinical trial.
Material and Methods
MUKseven is a randomised phase II study for relapsed or refractory myeloma (RRMM) patients comparing cyclophosphamide (500 mg po d1, 15, 21), pomalidomide and dexamethasone (CPd) versus standard pomalidomide and dex (Pd). Patients with ≥2 prior lines of therapy were randomised 1:1 to CPd or Pd and treated until disease progression. All patients underwent bone marrow sampling and peripheral blood collection, the latter for immune cell immunophenotyping at Cycle 1 Day 1 (C1D1; Baseline), C1D14 (on-treatment), C4D14 (on-treatment) and at disease progression. Peripheral blood (PB) T-cell populations were profiled using multicolor flow cytometry (MFC) designed to assess baseline and pharmacodynamic changes in subpopulations including helper, cytotoxic, naïve, memory, and activated/proliferating phenotypes (CD3, CD4, CD8, CD45RA, CD62L, HLA-DR, Ki67. T-cell sub-populations were defined and their respective % of total lymphocyte population used for downstream analyses.
Results
In total 102 evaluable RRMM patients were randomised, 51 each to CPd and Pd treatment arms, with comparable clinical baseline characteristics. Patients had received a median of 3 prior lines of treatment. Evaluable PB immune profiling data was available for 93 (91%) patients at Baseline, 83 (81%) at C1D14, 55 (54%) at C4D14 and 26 (25%) at progression.
We observed trends for changes in baseline T-cell population composition with increasing numbers of prior lines of therapy. Specifically, mean % CD4+ T-cells decreased from 35% for patients with 2 prior lines (n=18) to 30% with 3 (n=33), 23% with 4 and 20% with ≥5 prior lines of treatment (n=27), whilst the % of CD8+ cells were similar, indicating potential differential cumulative effects of anti-myeloma therapy on T-cell populations.
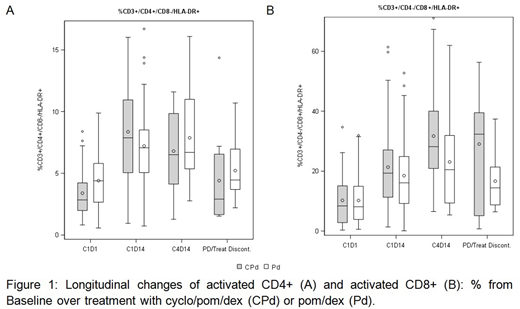
We compared changes in T-cell profiles longitudinally over trial treatment from baseline to C1D14 and C4D14 with summary statistics. Overall, there was a marked increase in activated (HLA-DR+) T-cells with therapy, with a 2-fold increase in mean proportion of activated CD4+ and CD8+ from 3.9% and 10.2% at baseline to 7.8% and 19.9% at C1D14 and 7.2% and 28.2% at C4D14, respectively (Figure 1). Trial treatment was associated with a shift in sub-populations within CD8+ T-cells in particular, with a relative % decrease in naïve (CD45RA+) sub-populations and increase in memory (CD45RA-) populations.
To identify differences associated with cyclophosphamide treatment a regression analysis was conducted on the C1D14 time point accounting for the treatment a patient received and incorporating their baseline (C1D1) measurement. The mean% estimates for total T-cells (CD3+) at C1D14 were significantly higher for the CPd arm in comparison to Pd: 72.1% [95% CI: 66.5 - 73.6] vs. 64.2% [58.2 - 66.1] (P=0.004). Estimates for the Pd arm appeared similar to baseline [mean C1D1: 61.7%]. Mean% estimates for CD8+ and CD4+ cells were also significantly higher with CPd treatment at C1D14 compared to Pd: 37.2% [32.6 - 38.0] vs. 33.1% [28.5 - 33.7] (P=0.03) and 26.2% [21.6 - 27.0] vs. 21.8% [21.6 - 27.0] (P=0.016), respectively. Importantly, mean% estimates for activated (HLA-DR+) CD4+ cells were significantly higher for the CPd arm 10.1% [6.9 - 9.4] vs 8.1% [5.1 - 7.2] in the Pd arm (P =0.02). There was a trend for additional increase of activated CD8+ cells by addition of cyclophosphamide to Pd therapy (P=0.06).
Discussion
We demonstrate for the first time in a randomised trial using systematic longitudinal immune profiling that addition of cyclophosphamide to pomalidomide and dexamethasone is significantly associated with altered T-cell profiles and an increased proportion of activated T-cells. MUKseven clinical endpoint data are reported separately, with improved response rates observed for CPd vs. Pd. Correlation of immune profiles with clinical outcomes and tumour genetics will be presented at the conference when PFS outcome data will be mature.
Boyd:Novartis: Consultancy, Honoraria; Janssen: Honoraria, Other: Travel and Accommodation expenses; Celgene: Consultancy, Honoraria, Other: Advisory role. Garg:Janssen: Honoraria; Novartis: Other: travel support, Research Funding; Takeda: Other: Travel Grant; Amgen: Honoraria, Other: Travel Support. Pawlyn:Janssen: Honoraria, Other: Travel support; Celgene Corporation: Consultancy, Honoraria, Other: Travel support; Amgen: Consultancy, Honoraria, Other: Travel Support; Takeda Oncology: Consultancy, Other: Travel support. Pierceall:Celgene: Employment, Equity Ownership. Cook:Celgene Corporation: Consultancy, Honoraria, Research Funding, Speakers Bureau; Sanofi: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Glycomimetics: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Seattle Genetics: Honoraria; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau. Thakurta:Celgene Corporation: Employment, Equity Ownership. Kaiser:Chugai: Consultancy; Takeda: Consultancy, Other: travel support; Celgene: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Other: travel support; Amgen: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal